
Radiation damage to Bowman-Birk soybean proteinase inhibitor was studied in dilute aqueous solutions containing: (a) air, (b) N/sub 2/O plus KBr, and (c) N/sub 2/O plus KCNS.
The irradiation of the inhibitor in the air saturated solution led to loss in tyrosyl as well as cystinl CD bands and decline in both antiproteinase activities.
CIRCULAR DICHROISM BUFFER CUT OFF WAVELENGTH FULL
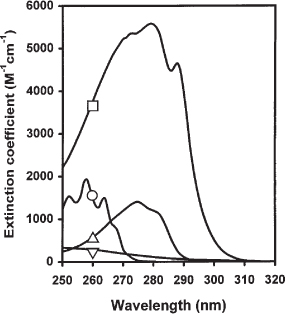
The inhibitor modified at Tyr 69 by Br/sub 2//sup -/ and (CNS)/sub 2//sup -/ retained full activity toward trypsin and chymotrypsin. Radical anions, Br/sub 2//sup -/ and (CNS)/sub 2/, generated by the irradiation of N/sub 2/O-saturated inhibitor solutions containing KBr or KCNS, reduced tyrosyl CD without affecting disulfide CD bands, indicating that the radical anions damaged Tyr 69 without altering protein conformation. the 232 nm more » positive shoulder is from the /sup 1/L/sub a/ vibronic transition of Tyr 69. the /sup 1/L/sub b/ vibronic transition of Tyr 69 has negative CD around 280 nm, contributing approximately 10% of the total CD intensity at 278 nm. The second cystinyl transition gives rise to a positive CD band of a comparable intensity at 247 nm. A broad negative CD band centered around 280 nm arises mainly from the longest wavelength transition of cystinyl side chains. Origins of CD bands in lima bean proteinase inhibitor were deduced from an acetylation-deacetylation study of the sole tyrosyl residue in the protein (Tyr 69), and by analogy with Bowman-Birk soybean proteinase inhibitor. Dithiothreitol (20 mM) completely abolished the tyrosyl and disulfide CD in this region. The near ultraviolet CD of BBI was altered in the presence of 8 M urea or 6 M guanidine hydrochloride, with a greater change brought about by the latter. Fully acetylated BBI has the near ultraviolet disulfide CD and the far ultraviolet polypeptide CD very similar to those more » of the native inhibitor, indicating that O-acetylation of two tyrosyl side chains did not induce much conformational change in BBI. Dimerization of monomeric BBI did not alter the CD profile. Tyrosyl side chains give rise to a sharp positive peak at 231 nm, overlapping with the positive disulfide CD. Disulfide bonds in BBI also have a broad positive CD band centered around 240 nm (epsilon/sub L/ - epsilon/sub R/ = 0.9 M/sup -1/cm/sup -1/ per disulfide). Each of two tyrosyl residues gives rise to negative CD at this region together they contribute approximately 10 percent of the total CD intensity at 277 nm (epsilon/sub L/ - epsilon/sub R/ = -0.36 M/sup -1/cm/sup -1/ per tyrosyl). A broad negative CD band centered around 280 nm in BBI arises mainly from disulfide bonds (epsilon/sub L/ - epsilon/sub R/ = -0.83 M/sup -1/cm/sup -1/ per disulfide). The O-acetylation of tyrosyl side chains showed that the ultraviolet CD bands of BBI above 225 nm originate from disulfide side chains and tyrosyl phenolic groups. The spectral properties of Bowman-Birk soybean trypsin inhibitor (BBI) were investigated by analyzing difference absorption spectra and difference circular dichroism (CD) spectra and by comparing them with those of tyrosyl model compounds.


 0 kommentar(er)
0 kommentar(er)
